Forensic Chemistry is a branch of chemistry that involves the application of chemical concepts to solve crimes.
Forensic chemistry is the application to analyze any non-biological materials found at a crime scene, and figure out how they connect to the person who committed the crime. It must determines the chemical makeup of the material and discover where it came from. A forensic chemist will perform various types of tests, depending on what the material is, to find where the material came from.
Types of cases encountered in Forensic Chemistry Division
- Cases related to NDPS drugs
- Trap cases
- Acid attacks
- Cases related to illicit liquor or beverages [alcoholic or non-alcoholic]
- Arson cases
- Cases related to cosmetics
- Adulteration
- Analysis of gold metal and alloys in cheating cases
- Examination of petroleum products such as diesel, kerosene, petrol.
- Analysis of low standard construction materials and also examination of adulteration.
- Examination of inflammable material in dowry cases.
There are particular standard operating protocols which are followed for the conformity of the drugs. It is followed as:


**Preliminary and presumptive test are same

PRELIMINARY EXAMINATION OR PRESUMPTIVE TEST
- This includes test just by looking at their physical properties which are not unique enough by themselves for identification but this provides enough information to narrow down the search.
- This analysis does not confirm about the substance but it narrow down the number of possibilities.
- This includes physical appearance [color and odor], pH testing, solubility, density, flame test.
- This test is done to analyze a sample and establish one of the following:
- The sample is definitely a X substance
- The sample is definitely not a X substance
CONFIRMATORY TEST
- These analyses include the properties which are unique to the substance for purpose of identification.
- This analysis confirms the substance absolutely.
- These analyses includes sophisticated instrumentation techniques to examine unique properties that’s leads to definite identification.
- These test are unequivocal.
CASES ENCOUNTERED IN FORENSIC CHEMISTRY
- Drug analysis:
Cases that involves drug abuse which has become a matter of concern and a serious threat. In this modern era, there has been a phenomenal rise in number of drug abuse cases. These cases includes identification of various drugs such as heroin, opium, morphine, barbiturates, cocaine etc. a proper standard of protocol is followed for their identification.
- Arson cases:
Examination of accelerant used during the arson case comes under this category. It involves the identification of cause of fire. It may be for the fraudulent insurance claim or to willfully causing destruction to property.
The physical evidence is in the form of burnt remains which can provide good deal of information. The burnt material will help to establish the cause of fire by identifying chemical composition of combustible agent used to set it alight and the consequences of the blaze.
- Trap Cases:
A trap is a method of deliberate setting of the bait and catch the accused person red handed. Phenolphthalein is being used in most of the anticorruption cases.A trap can be laid in 3 scenarios. To catch the bribe taker, to catch the bribe giver, and the cross-trap: to catch both, the bribe giver and the bribe taker. The third type of trap is usually done with an intelligent input from an independent third source.
- Adulteration:
Adulteration is the act of intentionally debasing the quality of food by adding or substituting low quality ingredients or by replacing active ingredient.
This is usually done to lower the cost or increase the bulk of a given food product. Adulteration is one of the common acts of black marketers and profiteers.
Adulteration is divided into two categories;

TECHNIQUES USED IN FORENSIC CHEMISTRY
- UV VISIBLE SPECTROSCOPY
Ultraviolet-visible (UV-Vis) spectroscopy is one of the most popular analytical techniques because it is very versatile and able to detect nearly every molecule. With UV-Vis spectroscopy, the UV-Vis light is passed through a sample and the transmittance of light by a sample is measured. From the transmittance (T), the absorbance can be calculated as
A=-log (T).
An absorbance spectrum is obtained that shows the absorbance of a compound at different wavelengths. The amount of absorbance at any wavelength is due to the chemical structure of the molecule. - UV-Vis can be used in a qualitative manner, to identify functional groups or confirm the identity of a compound by matching the absorbance spectrum. It can also be used in a quantitative manner, as concentration of the analyte is related to the absorbance using Beer’s Law.

- HIGH PERFORMANCE LIQUID CHROMATOGRAPHY
HPLC is a chromatographic technique similar to GC that involves the migration of a lipid mixture through a column containing a stationary phase. However, in HPLC the mobile phase is a liquid instead of a gas.
HPLC typically involves injecting a sample (20–200 μl) into an HPLC column while a mobile phase (solvent) is flowing through the column. The mobile phase can have many solvent combinations, but it typically contains water and an organic component.
The HPLC column is usually a stainless-steel tube ranging from 50 to 250 mm in length and 1–4.6 mm in diameter, packed with chemically modified silica particles (<1–5 μm in diameter) with a consistency of very fine sand.
The smaller the particle, the better the resolution of the mixture.
- Drugs – Many controlled substances are analyzed by HPLC. In addition drugs taken from body fluids can also be analyzed.
- Explosives – It may not be safe to run explosive extracts by GC because of the high heat, but HPLC is an ideal method for separation of explosive residues.
- LIQUID CHROMATOGRAPHY MASS SPECTROSCOPY
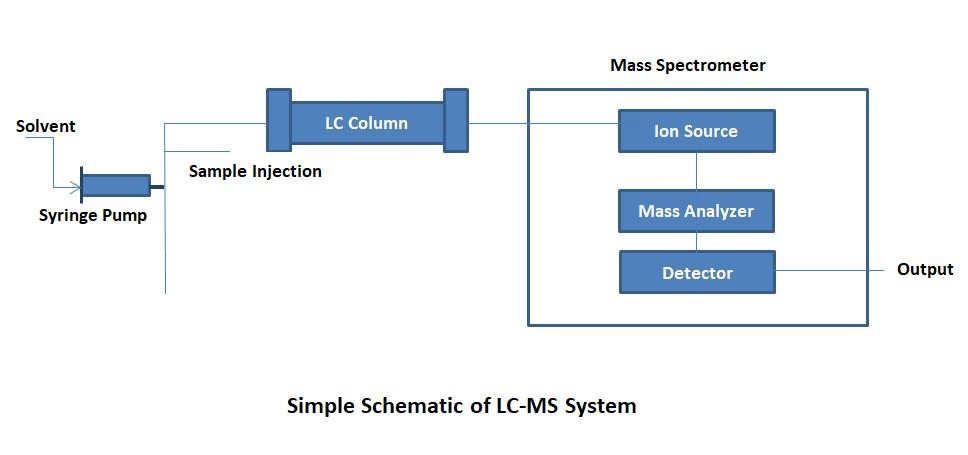
Liquid chromatography (LC) is a widely used method of sample ionization prior to analysis and is frequently coupled with mass spectrometry.
With LC-MS, solubilized compounds (the mobile phase) are passed through a column packed with a stationary (solid) phase.
This effectively separates the compounds based on their weight and affinity for the mobile and stationary phases of the column. This also leads to fragmentation of the sample and its anionization through loss of H+ ions.
It offers resolution and versatility. - The liquid chromatograph (LC) separates the components of a sample based on differences in their affinity for the stationary phase or mobile phase. Mass spectrometry (MS) offers a highly sensitive detection technique that ionizes the sample components, then separates the resulting ions based on their mass-to-charge ratios and measures the intensity of each ion. Therefore, LC-MS systems combine the separation resolution of liquid chromatography with the qualitative capabilities of mass spectrometry.
- GAS CHROMATOGRAPHY:
Gas chromatography differs from other forms of chromatography in that the mobile phase is a gas and the components are separated as vapors.
It is thus used to separate and detect small molecular weight compounds in the gas phase.
The sample is either a gas or a liquid that is vaporized in the injection port. The mobile phase for gas chromatography is a carrier gas, typically helium because of its low molecular weight and being chemically inert.
The pressure is applied and the mobile phase moves the analyte through the column. The separation is accomplished using a column coated with a stationary phase.

- AAS(ATOMIC ABSORPTION SPECTROSCOPY)
Atomic absorption spectroscopy has become one of the most frequently used tools in analytical chemistry. This is because for the determination of most metals and metalloids the technique offers sufficient sensitivity for many applications and is relatively interference free. - Atomic absorption spectroscopy is a commonly used technique for the determination of single elements in compounds. As the name suggests, the particles must be atomized in order to perform analysis. After the compound has been atomized (usually by a flame), a radiation source produces waves that pass through the substance and are received by a detector.
- ICP(INDUCTIVELY COUPLED PLASMA)
- The Inductively Coupled Plasma (ICP) is an ionization source that fully decomposes a sample into its constituent elements and transforms those elements into ions. It is typically composed of argon gas, and energy is “coupled” to it using an induction coil to form the plasma.
- NAA(NEUTRON ACTIVATION ANALYSIS)
- Neutron activation analysis (NAA) is a nuclear process used for determining the concentrations of elements in a vast amount of materials. NAA relies on excitation by neutrons so that the treated sample emits gamma-rays. It allows the precise identification and quantification of the elements, above all of the trace elements in the sample
- The method is based on neutron activation and therefore requires a source of neutrons. The sample is bombarded with neutrons, causing the elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element are well known. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the elements within it.
- A particular advantage of this technique is that it does not destroy the sample and is a sensitive technique.



Leave a Reply